Saddle Pulmonary Embolus
History of present illness:
A 29-year-old male presented to the emergency department with sudden onset dyspnea, which began earlier in the day and rapidly progressed, to the point that he cannot catch his breath. Past medical and surgical history is insignificant. The patient had a heart rate of 131, blood pressure of 124/73, and respiratory rate of 25 with a pulse oximetry of 92% on room air. Exam was notable for generalized pallor and clear lung sounds.
Significant findings:
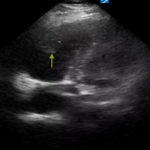
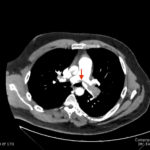
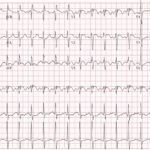
An electrocardiogram (ECG) showed evidence of right heart strain with an incomplete right bundle branch block, S1Q3T3 (see red arrow [S1], blue arrow [Q3], and black arrow [T3]), and ST-segment elevation in the septal leads (green arrows). Bedside echocardiography showed a dilated right ventricle with ventricular wall akinesis (red arrow) sparing the apex (purple arrow), which is known as McConnell’s Sign. It also showed a mobile hyperechoic mass (yellow arrow). These ultrasound findings were concerning for pulmonary embolism (PE), so computed tomography (CT) angiogram of the chest was ordered and confirmed massive bilateral obstructive filling defects (red arrows) consistent with saddle pulmonary embolism. Additionally, noted is flattening of the interventricular septum (blue arrow) consistent with right heart strain. Laboratory studies were notable for a troponin-I of 0.29 ng/mL, a B-type natriuretic peptide of 792.3 pg/mL, lactic acid of 5.30 mmol/L, and a creatinine of 2.0 mg/dL, consistent with end organ dysfunction. All other lab work was within normal limits.
Discussion:
Pulmonary embolus is a critical diagnosis that should be considered in every unstable dyspneic patient. With a large clot burden there is high risk of significant morbidity and mortality (30%-50%), including pulmonary infarct, pulmonary hypertension, and cardiac arrest.1-2 The use of electrocardiography helps screen patients for evidence of right heart strain. Findings may consist of sinus tachycardia, an S1Q3T3 pattern (specificity 97.7% and sensitivity 8.5%), right bundle branch block (specificity 97.7% and sensitivity 3.1%), T wave inversions in V1-3 (specificity 96% and sensitivity 10.5%), and ST segment elevation in the septal leads (specificity 92.6% and sensitivity 9.3%).3
Echocardiography is another useful tool in the bedside assessment of the dyspneic patient. In the case of pulmonary embolus, you should look for signs of right heart strain which include: abnormal right ventricular to left ventricular (RV:LV) ratio (specificity 97% and sensitivity 55%), right ventricle hypokinesis (specificity 91% and sensitivity 38%), and McConnell’s sign(specificity 95% and sensitivity 22%).4 With high pretest probability one can move to definitive testing or early involvement of consultants; CT angiography is currently the gold standard for ruling out pulmonary embolus due to its high sensitivity (97.3%) and rapid accessibility.5
Due to his significant clot burden and unstable vital signs, the case was discussed with interventional radiology who recommended thrombectomy, after which the patient had complete resolution of his symptoms. There are no formal indications for thrombectomy; however, most patients who undergo this procedure have a massive pulmonary embolus, extensive clot burden, or are unsuitable for systemic thrombolytics.6-8 Following thrombectomy, the patient was admitted to the medical intensive care unit on a heparin drip, was transitioned to coumadin, and discharged home several days later. His embolus was determined to be unprovoked. His work- up was negative for factor V Leiden mutation and antiphospholipid panel. His prothrombin gene mutation, protein C, and protein S levels were insignificant. At time of outpatient follow up he was well and had no complaints.
Topics:
Pulmonary embolus, Saddle PE, McConnell’s sign.
References:
- Becattini C, Agnelli, G, Lankeit, M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. doi: 10.1183/13993003.00024-2016
- Klok F, Zondag W, van Kralingen K, et al. Patient outcomes after acute pulmonary embolism. A pooled survival analysis of different adverse events. Am J Respir Crti Care Med. 2010;181(5):501-506. doi: 10.1164/rccm.200907-1141OC
- Marchick M, Courtney D, Kabrhel C, et al. 12-lead ECG findings of pulmonary hypertension occur more frequently in emergency department patients with pulmonary embolism than in patients without pulmonary embolism. Ann Emerg Med. 2010;55(4):331-335. doi: 10.1016/j.annemergmed.2009.07.025
- Ehrman R, Favot, M. Can echocardiography be used to diagnose pulmonary embolism at the bedside? Ann Emerg Med. 2018;72(3):310-311. doi: 10.1016/j.annemergmed.2017.09.018
- Mos IC, Klok FA, Kroft LJ, DE Roos A, Dekkers OM, Huisman MV. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7(9):1491-1498. doi: 10.1111/j.1538-7836.2009.03518.x
- Heberlein W. New generation aspiration catheter: feasibility in the treatment of pulmonary embolism. World J Radiol. 2013;5(11):430. doi: 4329/wjr.v5.i11.430
- Reekers J, Baarslag H, Koolen M, Van Delden O, van Beek, E. Mechanical thrombectomy for early treatment of massive pulmonary embolism. Cardiovasc Intervent Radiol. 2003;26(3):246-250. doi: 10.1007/s00270-003-1984-7
- Müller-Hülsbeck S, Brossman J, Jahnke T, et al. Mechanical thrombectomy of major and massive pulmonary embolism with use of the Amplatz thrombectomy device.Invest Radiol.2001;36(6):317-322. doi: 10.1097/00004424-200106000-00003