Right Ventricular Dilation in Patient With Submassive Pulmonary Embolism
History of present illness:
A 73-year-old female with a past medical history of tracheobronchomalacia, obstructive sleep apnea, diabetes, chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF) presented to the emergency department with four days of moderate to severe dyspnea on exertion. She endorsed right calf pain for the last few weeks and bilateral leg swelling, as well as productive cough, orthopnea, and non-radiating chest tightness but denied any hemoptysis. She had no history of thromboembolic disease, nor did she have any family history of clotting disorders. She was not currently on any anticoagulation. The patient lives a sedentary lifestyle at baseline. Upon arrival, her vital signs were as follows: temperature 97.3 F, pulse 60 per min, respirations 34 per min, blood pressure 113/85 mmHg, and oxygen saturation 97% on 4L nasal cannula. Physical exam was notable for jugular venous distension and bilateral lower extremity pitting edema. Lungs were clear to auscultation.
Significant findings:
Bedside echocardiography four chamber view revealed enlarged right ventricular (RV) to left ventricular (LV) ratio (greater than 1) on apical four-chamber view (see red and blue outlines respectively). The right atrium is not clearly delineated in this image and therefore is not outlined. One can also rule out a large pericardial effusion as the cause of her dyspnea, since there is no large hypoechoic collection surrounding the heart on either four- chamber view or parasternal long view.
Discussion:
Point of care ultrasound is a powerful tool that can aid in the diagnosis of undifferentiated dyspnea. Right ventricular size is one parameter that can signify right heart strain possibly due to an increase in resistance in the pulmonary vasculature. The normal RV should be approximately two-thirds the size of the left ventricle. Any RV between two-thirds and equal to the size of the LV is considered moderately dilated, and any RV greater in size than the LV is considered severely dilated.1,2 The RV to LV size ratio is best measured from the apical four-chamber view. Right ventricular shape will also change as afterload increases in the pulmonary circuit. As pulmonary vascular resistance increases, the RV (which has significantly less myocardium and thus greater compliance as compared to the LV) begins to lose its typical triangular shape and takes on a more rounded appearance.3 Likewise, the intraventricular septum becomes flattened during early diastole due to higher RV:LV pressures causing the LV to take on a “D” shaped appearance. These findings, collectively referred to as signs of “right heart strain,” typically improve with resolution of the clot burden.4
Unfortunately, while right ventricular dilatation and consequently an asymmetrical LV appearance are commonly encountered findings, they do not help distinguish acute from chronic right heart strain. Evaluation for McConnell’s sign, or right ventricular mid-free wall akinesia with RV apical sparing is important because it is considered to be the most specific finding (94%) for acute right heart strain.5
The decision to anti-coagulate empirically with heparin was delayed until the diagnosis could be confirmed with a CT angiogram of the chest. This patient had other underlying reasons for having a dilated RV (mainly her history of COPD, which can chronically increase afterload in the pulmonary circuit and require higher RV filling pressures to maintain cardiac output).6 As such, patients with COPD can develop cor pulmonale and exhibit right heart strain.7-9 Furthermore, this patient also had no clinical signs of deep venous thrombosis.
In a patient with no underlying disease that might cause a chronic RV dilation and no electrocardiographic evidence of RV ischemia or infarction (usually in association with an inferior myocardial infarction),10 a dilated RV on bedside echocardiogram in the acutely dyspneic patient should make one immediately suspicious for acute pulmonary embolism and may even prompt the clinician to begin empiric anticoagulation.
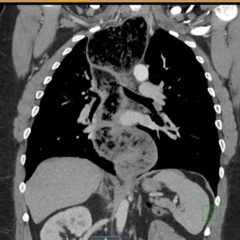
The patient’s lab work was significant for an elevated Troponin T of 0.03 ng/mL (normal ≤ 0.02 ng/mL), suggestive of right heart strain. The diagnosis of pulmonary embolism was confirmed on CT angiogram of the chest, which revealed large filling defects in the right and left pulmonary arteries extending into their respective segmental branches. Upon confirmation of the diagnosis, the patient was taken for emergent endovascular catheter-directed tissue plasminogen activator (tPA) and was subsequently admitted to the medical intensive care unit. She had an inferior vena cava (IVC) filter placed after five days and was discharged from the hospital seven days after admission.
Topics:
Pulmonary embolism, right heart strain, echocardiography, US, POCUS.
References:
- Acquah S, Arntfield R. Right Ventricular Function. Soni N, Arntfield R, Korry P, eds. Point of Care Ultrasound. First Edition. Philadelphia, PA: Elsevier Saunders; 2015:110-118.e2.
- Vieillard-Baron A, Prin S, Chergui K, et al. Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med.2002; 166:1310-1319. doi: 10.1164/rccm.200202-146CC
- Slama M, Maizel J, Mahjoub Y, El-Dash S. Evaluation of right ventricular function in the intensive care unit by echocardiography. Lumb P, Karakitsos D, eds. Critical Care Ultrasound. First Edition. Philadelphia, PA: Elsevier Saunders; 2015:179-184.e1
- Nass N, McConnell M, Goldhaber S, Chyu S, Solomon S.Recovery of regional right ventricular function after thrombolysis for pulmonary embolism. Am J Cardiol. 1999;83(5):804-806. doi: 10.1016/S0002-9149(98)0100-5
- McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-73. doi: 10.1016/S0002-9149(96)00339-6
- Falk J, Kadiev S, Criner GJ, Scharf SM, Minai OA, Diaz P. Cardiac disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):543-548. doi: 10.1513/pats.200708-142ET
- Burrows B, Kettel LJ, Niden AH, et al. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med. 1972; 286:912-917. doi: 10.1056/NEJM197204272861703
- Oswald-Mammosser M, Apprill M, Bachez P, Ehrhart M, Weitzenblum E. Pulmonary hemodynamics in chronic obstructive pulmonary disease of the emphysematous type. 1991; 58:304-310. doi: 10.1159/000195950
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. 2005; 127:1531-1536. doi: 10.1378/chest.127.5.1531
- Andersen HR, Falk E, Nielsen D. Right ventricular infarction: frequency, size and topography in coronary heart disease: a prospective study comprising 107 consecutive autopsies from a coronary care unit. J Am Coll Cardiol. 1987;10:(6)1223-1232. doi: 10.1016/S0735-1097(87)80122-5